HEALTH - LIBERTY - HAPPINESS
SUPPORT DOC MALIK HONEST HEALTH - BECOME A PAID MEMBER TODAY
Zero Corporate Sponsors - 100% supported by the People - Working For The People
The UK’s best podcast ;)
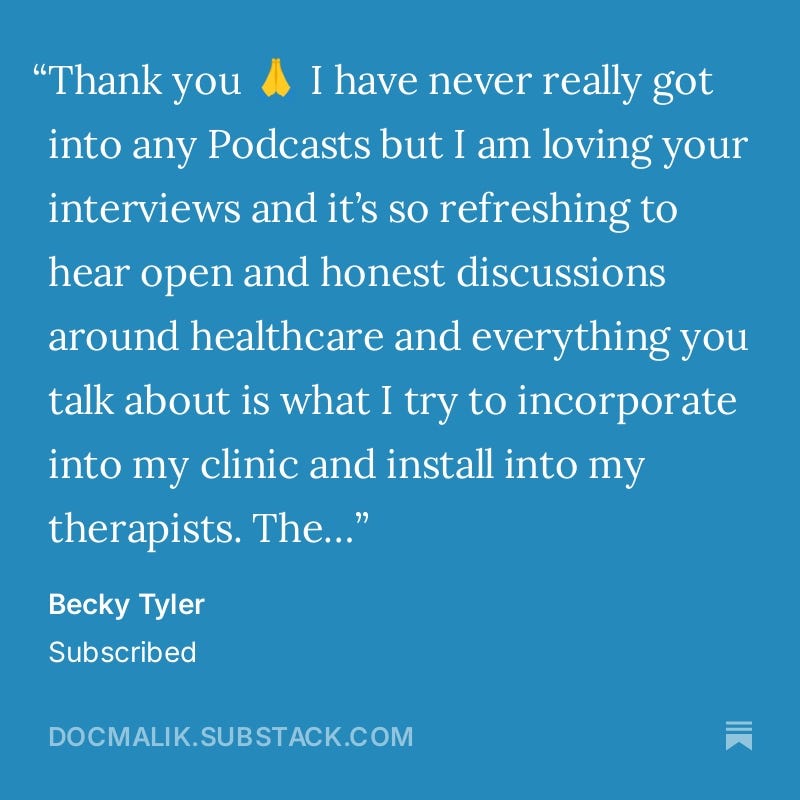
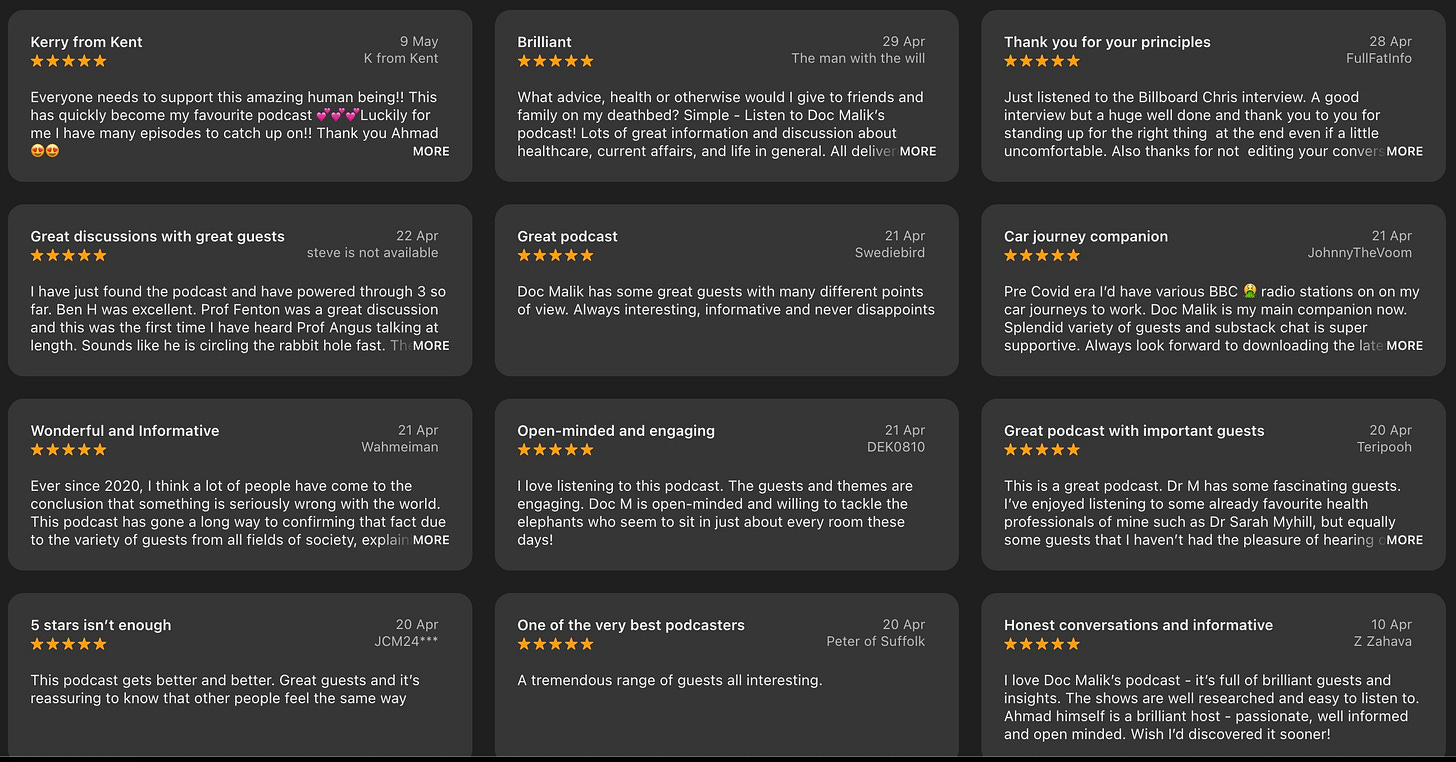
Thank you to all the new subscribers for your lovely messages and reviews! And a big thanks to my existing subscribers for sticking with me and continuing to support the show!
About this episode -
In this conversation, Andy and I dive into the impact of his film 'Protocol 7,' which exposes Merck's fraudulent practices around vaccine safety. We talk about the legal battles whistleblowers face, the collusion between big pharmaceutical companies and government agencies, and what all this means for public health. We also touch on the rise of autism, the corruption in scientific research, and how powerful film can be in bringing attention to these crucial issues. I share my own experiences as a medical professional who’s spoken out against the mainstream narrative, while Andy highlights the urgent need for accountability and transparency in the pharmaceutical industry.
We also discuss the complexities of vaccinology, the historical backdrop of vaccines, and how COVID-19 has shaped public health and filmmaking. We question vaccine effectiveness, explore the immune system, and discuss the flaws in vaccine safety studies. The conversation delves into how the pharmaceutical industry has influenced medicine and the personal journeys we've both been on as we navigate our roles in health and society.
Before you dive into the podcast.
Let’s just talk about the company Merck and what Protocol 7 is about.
Merck & Co Inc. is a multinational pharmaceutical company based in New Jersey, USA. Originally established as the American branch of Merck Group, which was founded in Germany in 1668, it has since become an independent entity. Today, Merck & Co. is one of the largest pharmaceutical companies globally, consistently ranking among the top five in revenue. In 2023, its revenue was a whopping 60 billion dollars!
Merck develops and produces medicines, vaccines, and biological therapies. You may have heard of many of these, such as the HPV vaccine Gardasil ($3.9 billion in revenue in 2020). It also has a chickenpox vaccine Variax ($1.9 billion in 2020), Pneumovax vaccine ($1.1 billion in 2020) and lesser known drugs such as Keytruda used in cancer immunotherapy ($14.3 billion dollars in 2020) and Janivia, a type 2 diabetic drug ($5.3 billion in 2020).
Vaccines and cancer treatment, as you can see, are big business and good for profits.
Maurice Hilleman, a scientist at Merck, developed the first mumps vaccine in 1967, the first rubella vaccine in 1969, and the first trivalent measles, mumps, and rubella (MMR) vaccine in 1971. Hilleman also created the first vaccines for Hepatitis B and chickenpox (varicella).
In 1979, Merck scientists developed lovastatin (Mevacor), the first Statin drug.
So, has Merck been up to any dodgy stuff in its history? Let's have a look…..
In 1999, the FDA approved Vioxx, a Merck drug for treating arthritis. Designed to reduce gastrointestinal (GI) side effects such as GI bleeding compared to older anti-inflammatory drugs, such as Naproxen, Vioxx became widely prescribed. As a young doctor, I remember the drug reps coming to our monthly departmental audit and teaching events, providing sandwiches and free pens. The consultants were all super excited about this new drug, Vioxx, and raved about it. I’m sure the free doughnuts had nothing to do with their enthusiasm for this drug or the fact that the reps were all young hot girls flirting with the senior consultants, no, never.
Merck executives were aware of potential cardiovascular risks from Vioxx as early as 2000 but continued its development and marketing. A 1999 clinical trial comparing Vioxx to naproxen showed fewer gastrointestinal issues but revealed nearly double the heart-related problems among Vioxx users. Despite the red flags, Merck chose not to study Vioxx’s heart effects, fearing it might harm its market position against its rival drug, Celebrex.
As Merck had hoped, results from the Vioxx Gastrointestinal Outcomes Research study (VIGOR) showed that patients taking Vioxx suffered fewer ulcers and bouts of gastrointestinal bleeding. But during the study, 79 of the 4,000 patients taking Vioxx suffered serious heart problems or died — a number nearly twice as high as in the naproxen group.
An estimated 88,000 Americans suffered heart attacks from Vioxx, with 38,000 deaths. More troubling information emerged in 2001, when Dr. Eric J. Topol and other cardiologists at Cleveland Clinic published a report in the Journal of the American Medical Association (JAMA) alleging that COX-2 inhibitors such as Vioxx appeared to increase the risk of cardiovascular events. Merck said the report was flawed and dismissed the call for a clinical trial explicitly aimed at investigating cardiovascular risks.
Topol reported that, before the report’s publication, scientists from the billion-dollar drug company requested that the information be kept from the public. Though the company changed Vioxx's label in 2002 to reflect heart attack risks, it wasn’t until 2004, after further studies confirmed stroke and heart attack risks, that Vioxx was withdrawn.
After Vioxx was eventually pulled from the shelves, Topol wrote an article published in the New England Journal of Medicine stating that “there may be tens of thousands of patients who have had major adverse events attributable to rofecoxib.” “Sadly, it is clear to me that Merck’s commercial interest in rofecoxib sales exceeded its concern about the drug’s potential cardiovascular toxicity”.
Interestingly, I don’t recall hearing my consultants discuss this or use it as a learning opportunity. I would have thought, considering what a fad this drug was in the recent past, this scandal would have been dissected by departments up and down the country and used as a warning to doctors of the problems (corruption and criminality) of Big Pharma, but it wasn’t.
Internal emails revealed that Merck had targeted doctors critical of Vioxx, seeking to "neutralise" or "discredit" them.
Merck faced several significant fines and settlements related to Vioxx:
In 2007, Merck settled heart attack injury cases for $4.85 billion without admitting fault.
In 2011, Merck paid the U.S. Department of Justice (DOJ) $950 million to resolve criminal charges and civil claims over illegal marketing of Vioxx. This included:
$321 million as a criminal fine for misbranding Vioxx.
$628 million for a civil settlement, split between the U.S. government and Medicaid states, over off-label marketing and false safety claims.
In a separate shareholder lawsuit, Merck agreed to pay $830 million to investors who claimed the company misled them about Vioxx's safety, leading to financial losses when the drug was withdrawn.
Despite these settlements, Merck continues to deny any wrongdoing.
Merck has paid a total of $4.85 billion to settle claims related to Vioxx victims, specifically for heart attacks and strokes caused by the drug. This settlement, reached in 2007, resolved around 50,000 lawsuits from individuals who suffered severe health problems after taking Vioxx. However, this settlement did not include victims outside the U.S., of which there are thousands, and Merck continues to deny any liability for the drug's harmful effects. What should also be recognised is that a significant chunk of this money went to pay legal fees. Most victims got peanuts.
Another shocking finding of the Vioxx scandal is that the FDA reviewers were aware of the potential for cardiovascular risk in 1999. It was argued that Merck had manipulated their ECG (EKG) tests one week after the external review board provided their consultation to expressly exclude high-risk factors from the trial subjects to avoid finding an effect, which then predated the changes to their trials to almost three months earlier.
So it that it? Sadly no. Between 2002 and 2005, Merck’s Australian branch paid Elsevier to publish eight Australasian Journal of Bone and Joint Medicine issues. Although it appeared to be an independent, peer-reviewed journal, Merck funded it, and favourable articles from other sources were reprinted without disclosing Merck’s involvement. This came to light in 2009 during a Vioxx-related personal injury lawsuit, where it was revealed that 9 of the 29 articles in one issue referenced Vioxx positively. The CEO of Elsevier’s Health Sciences Division later admitted the practice was “unacceptable.”
Let’s move to the next dodgy thing Merck has been involved in. Fosamax (alendronate) is a bisphosphonate used to treat post-menopausal osteoporosis and prevent skeletal issues in certain cancers. It is purported to reduce the risk of fractures by 35-39%.
In December 2013, Merck agreed to pay $27.7 million to 1,200 plaintiffs in a class action lawsuit claiming its osteoporosis drug, Fosamax, caused jaw osteonecrosis. Before the settlement, Merck had won 3 of 5 bellwether trials, with about 4,000 cases still pending.
What's also incredible is that for a drug that is meant to prevent osteoporosis (weak bones), Fosamax has been associated with an increased risk of thigh (femur) bone fractures, resulting in thousands of lawsuits. In 2022, Merck won the dismissal of 500 cases, but in 2024, an appeals court reversed that ruling. As of mid-2024, around 3,115 Fosamax lawsuits remained pending in U.S. courts.
If dodgy, unsafe drugs weren’t bad enough, Merck has also been found guilty of defrauding the government. In 2000, a fraud investigation by the U.S. Department of Justice began after whistleblowers claimed Merck failed to pay proper Medicaid rebates and made illegal payments to healthcare providers. In 2008, Merck settled the case for $650 million, one of the largest pharmaceutical settlements ever. Over $360 million went to the federal government, with $290 million distributed among 49 states and Washington, DC. A whistleblower received $68 million. You won’t be surprised to know, that Merck made the settlement without admitting any wrongdoing or liability.
Not happy with defrauding alone, Merck has added Tax evasion to its list of misdemeanours. In 2007, Merck paid $2.3 billion to settle allegations of offshore tax fraud between 1993 and 2001.
Good thing I like my bald head. In 2021, a Reuters investigation uncovered that Merck's baldness drug, Propecia, was linked to long-lasting sexual dysfunction in men. The drug has also been associated with over 700 cases of suicidal thoughts and 110 deaths. Despite receiving reports of these risks since 1998, Merck did not include warnings on the drug's label. Surprise, surprise, heh?
What about Gardasil? The HPV vaccine.
Safety Concerns: Reports of serious side effects (e.g., neurological issues).
Efficacy Questions: Concerns about false efficacy and effectiveness.
Mandatory Vaccination Policies: Laws requiring Gardasil for school entry spark debates on parental rights and equity in healthcare access.
Misleading Marketing Claims: Criticism that marketing exaggerates benefits and downplays risks.
Impact on Sexual Behavior: Concerns that vaccination may lead to riskier sexual practices.
Allegations of Conflict of Interest: Questions about financial ties between researchers, health organizations, and pharmaceutical companies affecting recommendations.
Honestly I am going to write a substack on Gardasil all on it’s own, it’s such a huge minefield.
So, this leads us to Protocol 7.
The Food and Drug Administration (FDA) is responsible for approving vaccines sold in the United States. In the 1960s, Merck received permission to sell its MMR vaccine, which combines mumps, measles, and rubella vaccines into one. For over fifty years, Merck was the only company allowed to sell this vaccine in the U.S. The vaccine has been updated over time, including the MMR-II vaccine licensed in 1978 and the ProQuad vaccine, which was licensed in 2005 and includes chickenpox protection.
The MMR-II vaccine has the same mumps and measles components as the original MMR vaccine but uses a different rubella vaccine. The ProQuad vaccine combines the MMR components with a chickenpox vaccine. Vaccines that protect against mumps, measles, and rubella are generally known as "MMR" vaccines, while those that also protect against chickenpox are referred to as "MMRV" vaccines. The FDA-approved labels for these vaccines provide information about their effectiveness and how well they trigger an immune response in recipients.
In the mid-1990s, the FDA began reviewing vaccine labels, including the MMR-II label. They determined that the vaccine’s potency should be measured at the end of its shelf life instead of when it was released. The FDA asked Merck to increase the minimum potency of its mumps vaccine to ensure that it remained effective when it expired. In 2000, Merck adjusted the potency of its vaccine but later received warnings from the FDA because some earlier vaccine lots did not meet the required potency standards.
In April 2010, a group of whistleblowers filed a complaint against Merck, alleging that the company had misled the CDC by omitting, concealing, and misrepresenting important information about its mumps vaccines. They claimed Merck had not been completely honest about the potency and effectiveness of the MMR-II and ProQuad vaccines, which could affect public health. The whistleblowers argued that Merck’s vaccine lots were sometimes released with lower-than-required potency levels, meaning that some doses might not provide sufficient protection against mumps.
Specifically, the complaints highlighted that Merck released many lots of the MMR-II vaccine that had lower potency than stated, especially before 2000, when the company began overfilling the vaccine to meet the potency requirements. Despite these concerns, Merck continued to sell the vaccine without adequately informing the FDA or the CDC about the issues. The whistleblowers also claimed that there was a culture at Merck that discouraged reporting negative findings and data manipulation in testing results.
In response to the complaints, the FDA conducted inspections and issued warnings to Merck about its practices. The whistleblower allegations included claims that raw data from studies was being changed, and procedures for ensuring the accuracy of lab testing were inadequate. As a result of these allegations, the FDA took a closer look at Merck's data and the methods used in testing vaccine effectiveness. This scrutiny led to ongoing debates about the vaccine’s actual effectiveness compared to what Merck had reported.
So what is Protocol 007?
1. Objective of Protocol 007: The primary aim of Protocol 007 was to support Merck's request for a label change that would allow the company to lower the end-expiry potency of its mumps vaccine from the established level of 4.3 log10 TCID50. To receive FDA approval for this change, Merck needed to demonstrate that even at lower potency, the vaccine could still provide sufficient protection against mumps.
2. Requirements for Approval: The FDA required that Merck show a seroconversion rate of at least 90% in groups receiving the lower potency doses. This threshold was crucial because it was the lower bound of a 95% confidence interval based on the previously established 96% seroconversion rate at the higher potency level.
3. Testing Methodology: Merck utilized two primary assays to evaluate the effectiveness of the vaccine:
- Plaque Reduction Neutralization Assay (PRN): This test assessed the ability of antibodies produced by vaccinated individuals to neutralize the mumps virus.
- Enzyme-Linked Immunosorbent Assay (ELISA): This assay measured the immune system's response to the vaccination.
4. Issues with the Testing: During the trials, while seroconversion rates for the Jeryl Lynn strain (the strain used in the vaccine) were high (91-96%), the rates for wild-type virus strains (the viruses circulating in the real world) were much lower, ranging from 56% to 76%. This raised concerns about the vaccine's effectiveness against actual mumps outbreaks.
5. Rabbit Antibody Use: In an effort to improve the seroconversion rates and support their application for a label change, Merck proposed using rabbit antibodies (specifically, anti-IgG) in the PRN tests. This involved altering the test methodology by incorporating these antibodies into the serum samples. This adjustment, referred to as the AIGENT assay, was intended to produce more favourable results regarding the vaccine's immunogenicity.
6. Data Integrity Concerns: There were serious allegations from employees in the lab conducting these tests regarding the integrity of the data. Reports indicated that raw data was being altered without justification and that there was an effort to fraudulently lower the pre-positive rates in the PRN assay. This manipulation was aimed at making it appear that more blood samples were seropositive (indicating a successful immune response) than actually were. In response to these allegations, the FDA conducted inspections of the lab, which led to further scrutiny.
7. Outcome of the Protocol: In 2004, the FDA ultimately did not approve Merck's application to change the vaccine's label based on Protocol 007. The agency found the information and data provided inadequate for final approval, particularly due to concerns regarding the methodologies employed, including the manipulation of data and the use of rabbit antibodies.
This whole affair shines a light on some big problems when figuring out how effective vaccines are. You know, it’s not just about making a vaccine and putting it out there; you’ve got to ensure the testing methods are solid and the data is trustworthy. That’s where things got a little shaky for Merck.
They started using rabbit antibodies in their tests, which sounds kind of strange, right? There were also some pretty serious allegations about manipulating the data to make the vaccine look better than it might actually be. This raises many eyebrows about whether Merck’s mumps vaccine is as safe and effective as it claims. It’s really important to have strict standards when it comes to testing and approving vaccines because we’re talking about people’s health here.
In short, the complaints against Merck are pretty concerning. Sadly, the legal system, regulatory bodies, and government have conspired to ensure that justice has not been served.
Here is the kicker: after digging into the evidence, both sides in the Merck case asked the court to make a decision without a trial. The court said no to the whistleblowers (the Relators) and yes to Merck. Even if Merck did make false claims about its vaccine, the court decided that those claims weren't important enough to affect the CDC's choice to buy the vaccine, which is necessary for holding Merck accountable. The court looked at a few key points from a previous case to figure this out.
First, the court noted that the CDC conducted its own studies on how effective the vaccine was. They found that the real-world effectiveness was lower than what Merck initially claimed, but they still said two doses of the vaccine were about 88% effective. Second, the court argued that even if there were misleading statements from Merck, they didn’t really change the overall deal with the CDC. The CDC continued to recommend the vaccine despite the ongoing legal issues. Lastly, the court believed that the government knew about the issues because they had access to the information shared during the investigation. Since they kept buying and recommending the vaccine anyway, it seemed like they weren’t too worried about those potential problems.
In short, the court found that even if Merck messed up, it didn’t matter enough for the CDC to change its mind about buying the vaccine, so they ruled in favour of Merck.
Now, just think about all the stuff you have read about Merck, how it has fiddled with figures, paid for journals, hidden damaging safety data, and being dishonest. If they have lied about all of this, what else have they lied about?
Can you really trust any products they sell?
I for one, think, frack that!
Enjoy my beautiful tribe
Ahmad x
Links
Website - https://protocol7.movie/
This is how I feel amongst the medical profession. Please sign up to my paid substack so that I can speak up for your freedom and fight the tyranny.
Health - Liberty - Happiness
Support Doc Malik
To subscribe to my paid substack - click here
To subscribe to my paid Spotify episodes - click here
To make a one time donation - Buy me a Coffee
For a regular coffee donation - click here
Remember after my cancellation I am wholly reliant on you, my listeners and readers to carry on my work speaking out for YOU and YOUR families.
Your contribution will make a difference.
You can also GIFT a subscription to a friend or family member - click here
Affiliates
As the number of paid subscribers grows slowly and gradually, I’ve had to find other ways to augment my income. I am only recommending products that I and my family already use.
Waterpure
We distil all our water for drinking, washing fruit and vegetables, and cooking. If you knew what was in tap water, so would you!
If you buy one please support me by using my code so I get £20 off. The code is www.waterpure.co.uk/docmalik
Make sure to put electrolytes back in your water. We use Hunter & Gather (see code below).
Hunter & Gather
Seed oils are inflammatory, toxic and nasty; eliminate them from your diet immediately.
If you want to pursue a keto or carnivore diet and avoid seed oils then I can’t recommend their products enough. Check out there website https://hunterandgatherfoods.com to see what they offer.
Use DOCHG to get 10% OFF your purchase (so buy a ton load)
I get 10% on all sales, so please buy a lot (hee hee).
MERCHANDISE
You also have 10% off all my merch using the code - DOCMALIKSUPPORTER
To all the new subscribers welcome to the Clan!
Please make sure to check your emails download the Substack app so that you can join the chat function.
And oh yeah thank you for the coffee folks x
I hope you enjoy this episode.
YOU ARE BEAUTIFUL AND AWESOME
Much love Ahmad
Disclaimer
I Ahmad Malik: am a private civilian, protected by the Geneva Convention.
My substack, social media posts and podcasts are my personal experiences, observations and opinions. This information is for educational purposes only. Although I am a doctor, I am not your doctor, and I am not providing medical or legal advice to you or to the wider public. I am not licensed or registered with the GMC or any other licensing board.
The responsibility for the interpretation, due diligence and use of the information from my substack and my podcast lies with you, the viewer and/or listener. Please do your research, and use your discernment.
It is not my intention to harass, intimidate, offend, defame, conspire, blackmail, coerce or cause anxiety, alarm or distress to any man or woman, and the information presented here is done so with peaceful and honourable intentions.
Private Rumble link below
Listen to this episode with a 7-day free trial
Subscribe to Doc Malik Honest Health to listen to this post and get 7 days of free access to the full post archives.